Homework 1
ECE 4339
Han Q. Le
(copyrighted) U. of Houston
1. (20 pts) Graphene lattice and crystal structure
Use the app in lecture to obtain a graphics of graphene. You can search on the Internet for any graphics of graphene crystal and have them compared. Specifically: How many carbon atoms are there in each unit cell? What are their relative coordinates within a unit cell?
2. (20 pts) Some crystal structures
Use those links to Wolfram demonstration projects to obtain a graphic for each crystal below (you generate, then copy and paste):
2.1 Si crystal (diamond structure)
2.2 GaAs or any III-V semiconductor with zincblende structure
3. (10 pts) Crystal growth
Watch those youtube clips cited in the lecture or select your own about how large single Si crystal ingots (or boules) are grown, then write a few paragraphs (1/3 -1/2 of a page) to summarize what you observed.
4. (20 pts) Basic review about light.
4.1 (5 pts) Speed of light
The speed of light c is ![]() meter/sec. Express the speed of light in the following units:
cm/sec; μm/ps; where:
meter/sec. Express the speed of light in the following units:
cm/sec; μm/ps; where: ![]() .
Try to remember the value of c in μm/ps. It is a very
useful unit.
.
Try to remember the value of c in μm/ps. It is a very
useful unit.
4.2 (5 pts) Frequency
Calculate the frequency of light in unit of THz
(Terahertz), which is ![]() Hz, using the result of c in the unit of μm/ps from
question 1 above, for the following wavelengths: 0.78 μm (the
wavelength of CD laser), 0.65 μm (the wavelength of DVD laser),
0.633 μm (HeNe laser wavelength), 0.532 μm (the wavelength of
doubled Nd:YAG, such as green laser pointer ), and 0.405 μn
(BluRay laser). What is the color of each wavelength? (search for
picts that shows colors of these wavelength on the Internet).
Hz, using the result of c in the unit of μm/ps from
question 1 above, for the following wavelengths: 0.78 μm (the
wavelength of CD laser), 0.65 μm (the wavelength of DVD laser),
0.633 μm (HeNe laser wavelength), 0.532 μm (the wavelength of
doubled Nd:YAG, such as green laser pointer ), and 0.405 μn
(BluRay laser). What is the color of each wavelength? (search for
picts that shows colors of these wavelength on the Internet).
4.3 (10 pts) λ vs. f
Plot lightwave frequency (in unit of THz) as a function of wavelength (in unit of μm) on log-log scale for λ (wavelength) from 10 μm to 0.1 μm.
Answer given (but you must import and paste into your work - unless you use Mathematica, then you can leave as is)
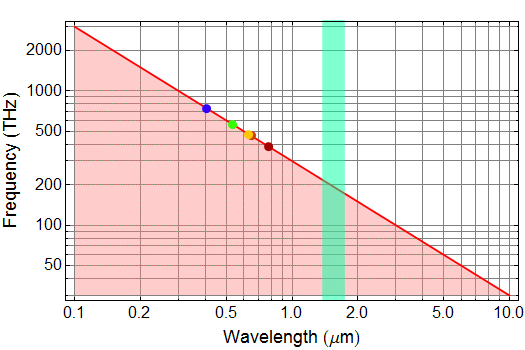
5. (30 pts) photon quantum energy and units.
5.1 (5 pts) Quantum
We discuss Planck’s light quantum, the smallest
anount of energy that a lightwave carries as: E=h ν
where h is Planck’s
constant and ν is the light frequency. (h= 6.62606896 ![]() Joule/s). Find the photon energy for each laser light in question
4.2 in Joule
Joule/s). Find the photon energy for each laser light in question
4.2 in Joule
Answer
5.2 (5 pts) The unit of eV
A most useful unit of energy is eV= electron-Volt:
it is the energy to move an electron across a potential of 1 V.
What is one eV in the unit of Joule. (note the charge of an
electron is: e=1.602176487 ![]() Coulomb.
Coulomb.
Answer
5.3 (5 pts) Question 6: photon energy
What is the energy of each photon in question 5.1 in unit of eV?
Answer
5.4 (5 pts) Question 7: photon energy vs. λ
Plot the photon energy (in unit eV) as a function of wavelength on log-log scale for λ (wavelength) from 10 μm to 0.1 μm.
Answer given (but you must import and paste into your work - unless you use Mathematica, then you can leave as is)
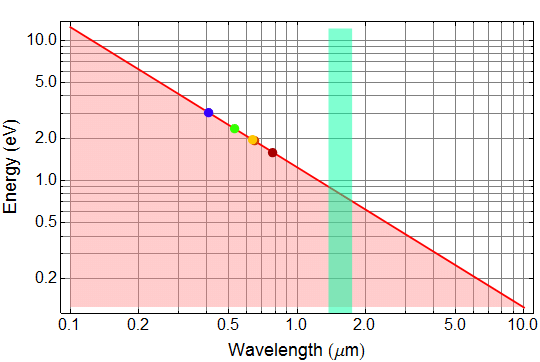
5.5 (10 pts) Astronomical gamma-ray energy
This is an exercise in unit conversion. Below is the plot of gamma ray intensity as function of photon energy from the center of our galaxy (Milkyway) obtained by Max Planck Institute for Astrophysics. (γ-ray is photon with very high energy.)
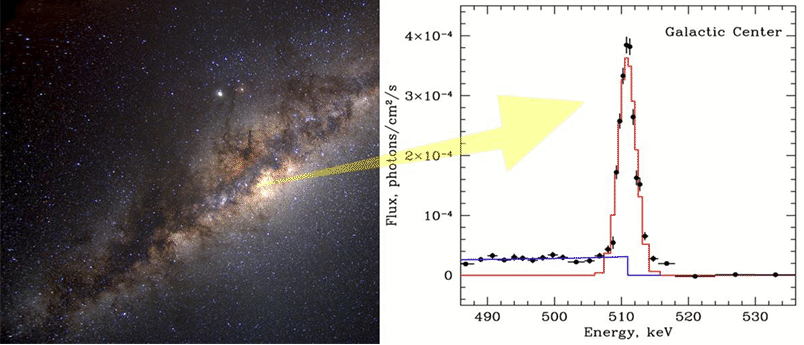
Using the formula: ![]() ,
and the electron rest mass is 9.10938215
,
and the electron rest mass is 9.10938215![]() Kg, guess what nature of the peak at 511 keV (kilo eV) in the
graph is. Calculate the 511-keV-line intensity (the peak if the
graph) in unit of
Kg, guess what nature of the peak at 511 keV (kilo eV) in the
graph is. Calculate the 511-keV-line intensity (the peak if the
graph) in unit of ![]() Hint, look up matter-antimatter, and electron its anti-matter.
Hint, look up matter-antimatter, and electron its anti-matter.